LINGZHI MAY AMELIORATE DRUG-INDUCED HEPATOTOXICITY WU TINGYAO
Chemotherapy injures the liver and kidneys while Lingzhi (also called Ganoderma lucidum or Reishi mushroom) protects the liver and kidneys.
Can Ganoderma lucidum withstand the liver and kidney damage caused by chemotherapy?
A team composed of Professor Hanan M Hassan from the Faculty of Pharmacy of Delta University for Science & Technology in Egypt and Professor Yasmen F Mahran from the Faculty of Pharmacy of Ain Shams University in Egypt used cisplatin, the most common traditional chemotherapy drug, to test the possibility of Ganoderma lucidum in protecting liver and kidney cells from cisplatin injury.
Their research results are divided into two articles: one is protecting the liver while another is protecting the kidneys. They were published in “Drug Design, Development and Therapy” and “Oxidative Medicine and Cellular Longevity” in June and July 2020, respectively.
The anti-oxidant, anti-inflammatory and anti-apoptotic effects of Ganoderma lucidum can obviously block many oxidative damage, inflammatory damage and cell apoptosis induced by cisplatin, and such protection is applicable to liver cells or kidney cells. This not only highlights the dual medicinal value of Ganoderma lucidum but also provides a feasible auxiliary protection method for cancer chemotherapy.
In order to avoid making this article too long, the author will introduce the role of Ganoderma lucidum in this aspect in two parts in the hope that these scientifically based data and evidence will bring more confidence to friends who seek to reduce the side effects of chemotherapy.
Part 1 Ganoderma lucidum protects the liver vs. cisplatin hepatotoxicity
The researchers compared the differences between using and not using Ganoderma lucidum during cisplatin treatment in six groups of healthy rats and the differences in protection against liver injury with different Ganoderma lucidum administration methods. They are:
◆Control Group (Cont): the group that does not receive any treatment;
◆Ganoderma lucidum Group (GL): the group that are not injected with cisplatin but eats Ganoderma lucidum every day;
◆Cisplatin Group (CP): the group that is only injected with cisplatin but does not eat Ganoderma lucidum;
◆Everyday Group (Daily): the group that is injected with cisplatin and eats Ganoderma Lucidum every day;
◆Every Other Day Group (EOD): the group that is injected with cisplatin and eats Ganoderma lucidum every other day;
◆Intraperitoneal Group (i.p): the group that is injected cisplatin and receives intraperitoneal injection of Ganoderma lucidum.
All those who received cisplatin were injected intraperitoneally with 12 mg/kg of Cisplatin on the first day of the experiment to trigger acute liver injury; those who received the intraperitoneal injection of Ganoderma lucidum were injected once on the second and sixth days of the experiment.
The Ganoderma lucidum used in the experiment contains active ingredients such as triterpenes, sterols, polysaccharides, polyphenols and flavonoids. The Ganoderma lucidum given in animal experiments, whether it is taken orally or by injection, is calculated at a daily dose of 500 mg/kg.
(1) Ganoderma lucidum reduces hepatocellular injury
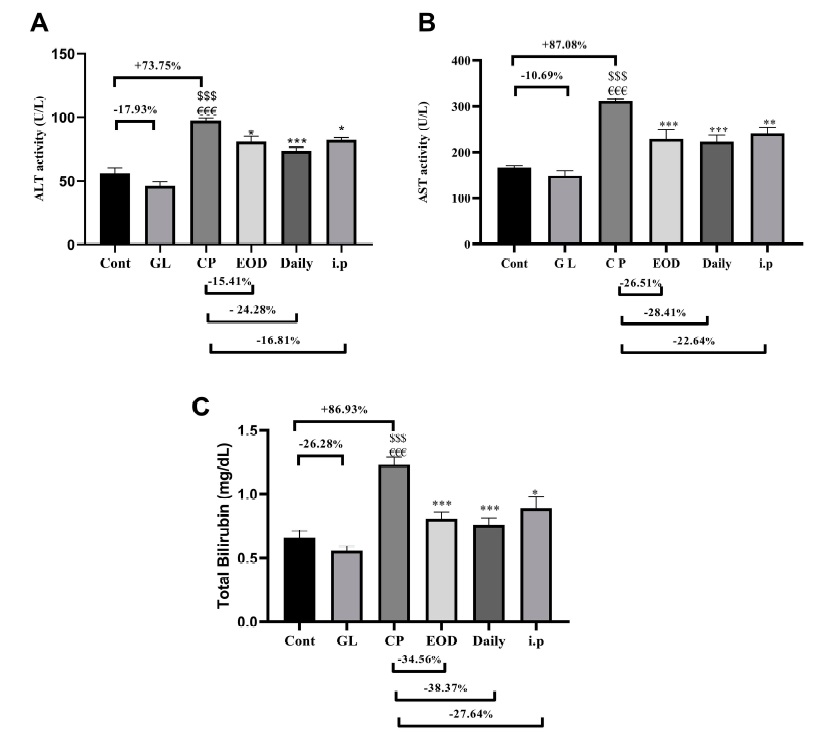
After 10 days, it can be seen that cisplatin will increase the hepatitis index and total bilirubin level in the rat’s serum. These are all signs of hepatocellular injury. But if Ganoderma lucidum is involved at the same time, the increased value can be reduced a lot (Figure 1).
Data Source/Drug Des Devel Ther. 2020; 14:2335-2353.
Figure 1 Effects of cisplatin and Ganoderma lucidum on liver injury indicators
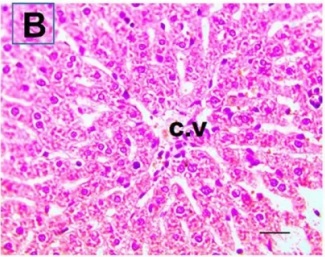
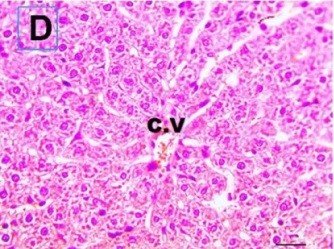
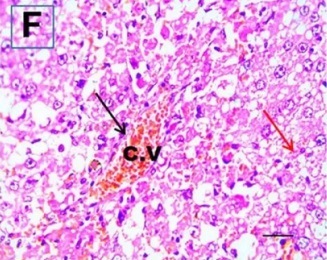
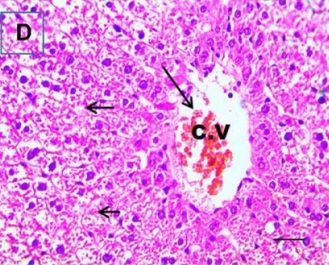
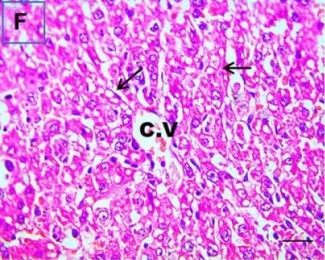
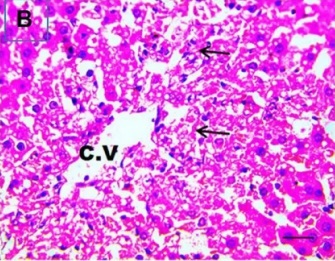
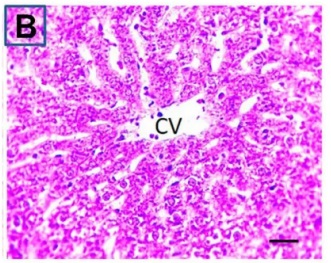
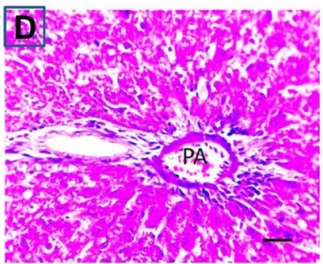
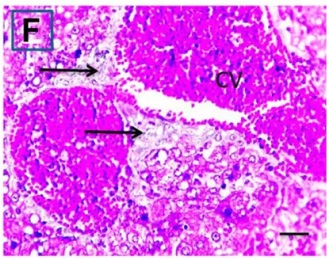
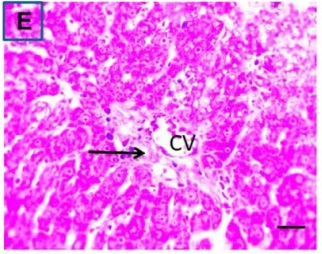
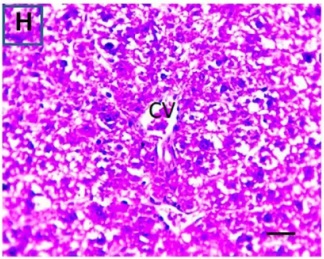
Put the liver tissue section under a microscope, and you can see that cisplatin can cause liver congestion (the blood that should return to the heart is blocked and stagnates in the hepatic veins), cell degeneration (vacuoles appear, which is the earliest change in cellular injury), apoptosis and necrosis, but these conditions can also be alleviated by using Ganoderma lucidum.
|
Control Group (Cont) |
Ganoderma lucidum Group (GL) |
|
Cisplatin Group (CP) |
Every Other Day Group (EOD) |
|
Everyday Group (Daily) |
Intraperitoneal Group (i.p) |
| C.V. refers to the central vein.The arrows point to areas of hepatic congestion or hepatocyte degeneration. Data Source/Drug Des Devel Ther. 2020; 14: 2335-2353. |
|
Figure 2 Effects of cisplatin and Ganoderma lucidum on hepatocytes
(2)Ganoderma lucidum enhances the antioxidant capacity of liver cells
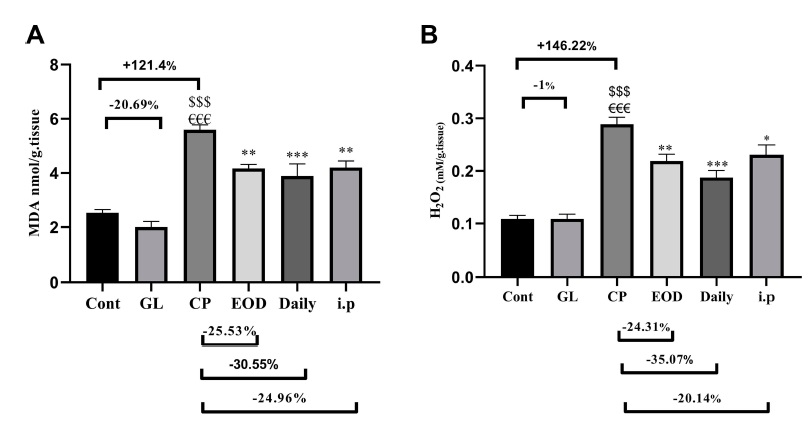
This article further compares the oxidative damage suffered by each group of liver tissues. There are two observation indicators: MDA (malondialdehyde), a product formed after the destruction of cell membranes by free radicals, and H2O2 (hydrogen peroxide), an intermediate product formed after the metabolism of free radicals by antioxidant enzymes.
Both of these products have the oxidative properties of free radicals and must be further treated before they can be truly “detoxified”, so the amount of them can tell us the oxidative damage that the liver tissue “has suffered” and “will suffer”.
Obviously, cisplatin will cause great oxidative damage to liver tissue, but if Ganoderma lucidum is involved In treatment at the same time, such damage can be reduced (Figure 3).
Because the changes in the concentration of antioxidant enzymes (SOD and GSH) in the liver tissues of each group and the changes in oxidative damage indicators showed a completely opposite trend, it can be inferred that Ganoderma lucidum will increase the antioxidant capacity of liver tissue and reduce the damage by “increasing antioxidant enzymes”.
Figure 3 Effects of cisplatin and Ganoderma lucidum on oxidative damage of liver tissue
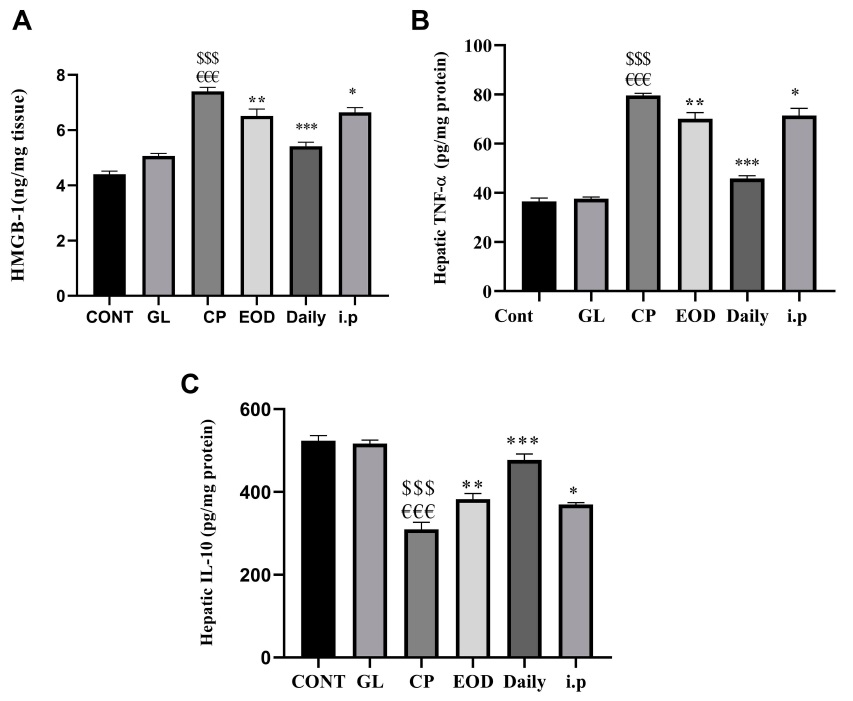
(3)Ganoderma lucidum enhances the anti-inflammatory ability of liver cells
Cisplatin threatens the survival of cells by damaging DNA and inducing a large number of free radicals; cells under pressure will turn on the master switch NF-kB that regulates the inflammation response, prompting cells to synthesize and release tumor necrosis factor (TNF-α) and other cytokines to activate the first wave of inflammatory reactions and sound the alarm for immunity.
Immediately afterwards, those cells killed by oxidative damage or inflammation will release another cytokine, HMGB-1, to activate more immune cells, triggering waves of inflammation.
Continuous inflammation will not only, in turn, intensify oxidative damage but also drive more cells to death, and even cause liver tissue to gradually develop fibrosis during the process of repeated inflammation and repair.
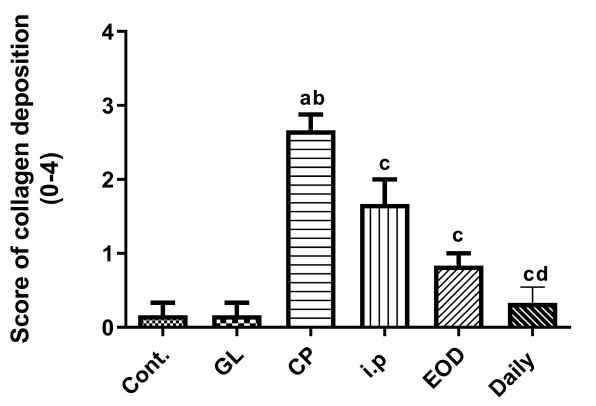
Fortunately, just like Ganoderma lucidum can reduce the oxidative damage caused by cisplatin, animal experiments also confirmed that the combined use of cisplatin and Ganoderma lucidum can inhibit the activation of the inflammation switch NF-kB, reduce the inflammation-promoting TNF-α and HMGB-1, and increase the anti-inflammatory cytokine IL-10 in the tissues of the liver at the same time (Figure 4).
Taken together, these effects not only inhibit inflammation but also reduce collagen deposition and prevent the progression of liver fibrosis (Figure 5).
Data Source/Drug Des Devel Ther. 2020; 14: 2335-2353.
Figure 4 Effects of cisplatin and Ganoderma lucidum on inflammation of liver tissue
|
Control Group (Cont) |
Ganoderma lucidum Group (GL) |
|
Cisplatin Group (CP) |
Every Other Day Group (EOD) |
|
Everyday Group (Daily) |
Intraperitoneal Group (i.p) |
|
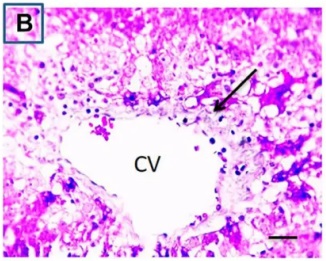
The arrows point to areas of collagen deposition. |
|
Data Source/Drug Des Devel Ther. 2020; 14: 2335-2353.
Figure 5 Effects of cisplatin and Ganoderma lucidum on liver fibrosis
(4)Ganoderma lucidum enhances the anti-apoptotic ability of liver cells
Whether through oxidative damage or inflammatory damage, cisplatin will eventually activate the “apoptosis” mechanism and force liver cells to die.
In other words, if liver cells can hold the last line of defense, they will have more chances to survive and reduce the severity of liver damage.
There are many protein molecules that regulate apoptosis. Among them, the most representative ones are: p53, which can promote apoptosis, Bcl-2, which can inhibit apoptosis, and caspase-3, which executes apoptosis at the last minute.
According to the researchers’ analysis of liver tissues of experimental animals in each group, Ganoderma lucidum can not only promote the expression of Bcl-2 but also inhibit the expression of p53 and caspase-3, which can provide powerful anti-apoptotic energy for liver cells.
(5) Ganoderic acids play an important anti-inflammatory role
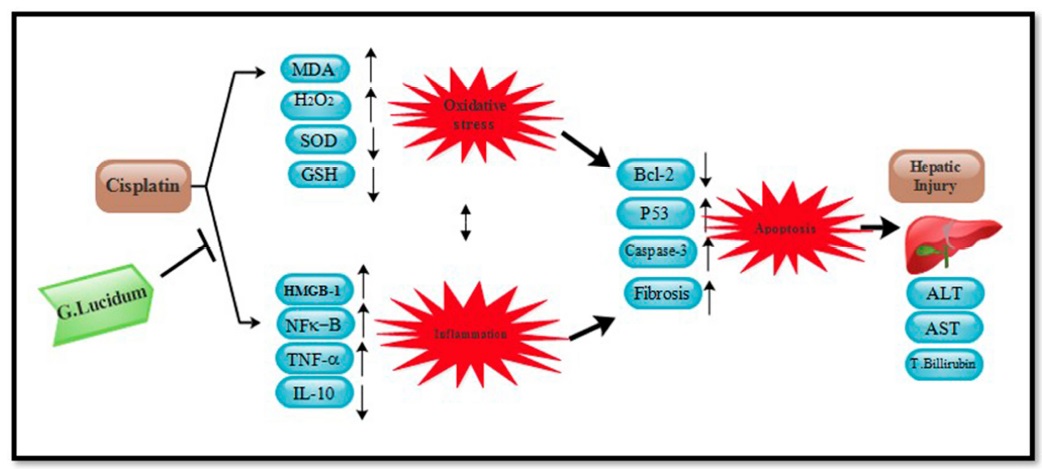
From anti-oxidation, anti-inflammation, anti-apoptosis to the actual performance of reducing liver damage, the researchers have compiled the mechanism of Ganoderma lucidum in inhibiting cisplatin hepatotoxicity into the following diagram for your reference.
Data Source/Drug Des Devel Ther. 2020; 14: 2335-2353.
Figure 6 The mechanism of Ganoderma lucidum in inhibiting liver toxicity of cisplatin
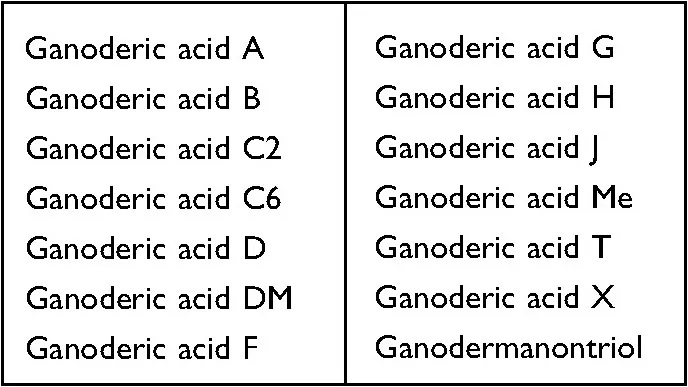
In the end of this study, the analysis of the “molecular docking simulation system” found that at least 14 ganoderic acids in the triterpenes of Ganoderma lucidum (as shown in the table below) can directly and effectively bind to the key cytokine HMGB-1, thus inactivating the pro-inflammatory activity of HMGB-1.
Since anti-inflammation is one of the important mechanisms of Ganoderma lucidum to reduce Cisplatin-induced hepatotoxicity, “richness in Ganoderic acid” has become an indicator component of Ganoderma lucidum to protect the liver.
What kind of Ganoderma lucidum ingredients can contain such abundant Ganoderic acids? According to past research, it is known that they are mainly present in the “Ganoderma lucidum fruiting body alcohol extract”.
It is worth mentioning that the rats in the Ganoderma lucidum group that only ate Ganoderma lucidum are almost the same as the rats in the control group in the above-mentioned experimental results, indicating that Ganoderma lucidum is highly safe for consumption.
In addition, the method of using Ganoderma lucidum is also very important. If you are willing to review the chart shown in this article, it is not difficult to find that “Every Day Group” has the best effect.
In fact, Every Day Group has the best effect in reducing the hepatic and renal toxicity of cisplatin in animal experiments,which is different from other Ganoderma lucidum groups.
What are the specific manifestations of the above-mentioned good effects? Stay tuned for “Part 2 Ganoderma lucidum protects the kidney vs. Cisplatin nephrotoxicity”.
[Data Source]
1.Hanan M Hassan, et al. Suppression of Cisplatin-Induced Hepatic Injury in Rats Through Alarmin High-Mobility Group Box-1 Pathway by Ganoderma lucidum: Theoretical and Experimental Study. Drug Des Devel Ther. 2020;14: 2335-2353.
2.Yasmen F Mahran, et al. Ganoderma lucidum Prevents Cisplatin-Induced Nephrotoxicity through Inhibition of Epidermal Growth Factor Receptor Signaling and Autophagy-Mediated Apoptosis. Oxid Med Cell Longev. 2020. doi: 10.1155/2020/4932587.
END
About the author/ Ms. Wu Tingyao
Wu Tingyao has been reporting on first-hand Ganoderma lucidum information since 1999. She is the author of Healing with Ganoderma (published in The People’s Medical Publishing House in April 2017).
★ This article is published under the exclusive authorization of the author, and the ownership belongs to GANOHERB ★ The above works cannot be reproduced, excerpted or used in other ways without the authorization of GanoHerb ★ If the works have been authorized to be used, they should be used within the scope of authorization and indicate the source: GanoHerb ★ Violation of the above statement, GanoHerb will pursue its related legal responsibilities ★ The original text of this article was written in Chinese by Wu Tingyao and translated into English by Alfred Liu. If there is any discrepancy between the translation (English) and the original (Chinese), the original Chinese shall prevail. If readers have any questions, please contact the original author, Ms. Wu Tingyao.